Table of Contents
- 1.1 Purpose
- 1.2 Intended Audience
- 1.3 History of the USPSTF
- 1.4 Scope of Work
- 1.5 USPSTF Members
- 1.6 USPSTF Meetings
- 1.7 Conflict of Interest
- 1.8 Partner Organizations
- 1.9 Overview of the Process
- 1.10 Procedures for Writing Papers and Documents
1.1 Purpose
The U.S. Preventive Services Task Force's (USPSTF's) mission is to improve the health of people nationwide by making evidence-based recommendations about clinical preventive services and health promotion.
This Procedure Manual documents the methods used by the Task Force to ensure that its recommendations and the reviews on which they are based are of consistently high quality, methodologically sound, scientifically defensible, reproducible, unbiased, and well documented.
The USPSTF is assisted in fulfilling its mission by the Agency for Healthcare Research and Quality (AHRQ), which provides scientific, administrative, and dissemination support to the USPSTF, and by AHRQ-designated Evidence-based Practice Centers (EPCs), which develop the evidence reviews, evidence summaries, and other documents that inform the USPSTF's deliberations. In addition to documenting the USPSTF's methods, this Manual also provides a summary overview of the methods used by AHRQ and EPC staff to support the USPSTF.
1.2 Intended Audience
The Procedure Manual is a user's manual for everyone on the USPSTF team—including AHRQ and EPC staff in addition to Task Force members. It is designed primarily for internal use as a guide to developing USPSTF recommendations, but may also be of interest to researchers, methodologists, and members of the public. It is intended to be a "living" document that is constantly updated as methods and processes evolve.
In developing this Manual, the Task Force drew, in part, from a series of articles published by its members, past members, AHRQ staff, and other researchers. A list of these sources is provided in Section 10. Researchers and methodologists seeking further details on the Task Force's methodology may find these articles useful as a complement to the Manual.
1.3 History of the USPSTF
The USPSTF, first convened by the U.S. Public Health Service in 1984, is a leading independent panel of nationally recognized non-Federal experts in prevention and evidence-based medicine. Programmatic support for the Task Force was transferred to AHRQ in 1995. The Affordable Care Act of 2010 reauthorized the USPSTF with a slightly different and expanded mandate. Due to the Nation's greater emphasis on prevention, insurers are now required to cover preventive services that are recommended by the USPSTF with a grade of A or B, along with those recommended by the Centers for Disease Control and Prevention's (CDC's) Advisory Committee on Immunization Practices (ACIP), Bright Futures, and the Health Resources and Services Administration's (HRSA's) guidelines for women's health. The Affordable Care Act requires insurers to cover these services with no deductible and no co-pay (Appendix I).
The first Task Force concluded its work in 1989 with the publication of the "Guide to Clinical Preventive Services." A second Task Force, appointed in 1990, concluded its work with the release of the second edition of the "Guide to Clinical Preventive Services" in December 1996. In 1998, members of the third Task Force were appointed for 5-year terms. The third Task Force released its recommendations incrementally.
Since 2001, the Task Force has featured a rolling panel of members appointed for 4 years, with a portion of the membership being replaced each year. Additionally, Task Force methods were described in a special issue of the American Journal of Preventive Medicine that year, including methods for developing recommendations on behavioral counseling and use of analytic frameworks. (See Section 10 for reference.) Following this publication, the Task Force began systematically using analytic frameworks to structure literature reviews and develop recommendations on every topic.
The Task Force now releases its recommendations both incrementally and in periodic publications similar to the "Guide to Clinical Preventive Services."
1.4 Scope of Work
Since its inception almost 30 years ago, the USPSTF has worked to fulfill its mission of improving the health of all Americans by making evidence-based recommendations about clinical preventive services and health promotion.
The Task Force comprehensively assesses evidence and makes recommendations about the effectiveness of clinical primary and secondary preventive services, including screening tests, counseling about healthful behaviors, and preventive medications for children, adolescents, adults, older adults, and pregnant women.
Its recommendations focus on interventions to prevent disease, so they only apply to persons without signs or symptoms of the disease or condition under consideration. USPSTF recommendations address services offered in the primary care setting or services referred by primary care professionals.
While the main audience for Task Force recommendations is the primary care clinician, the recommendations also have relevance for and are widely used by policymakers, managed care organizations, public and private payers, quality improvement organizations, research institutions, and patients.
1.5 USPSTF Members
There are currently 16 members on the Task Force. Members are nationally recognized experts in prevention, evidence-based medicine, and primary care who are also skilled in the critical evaluation of research and the implementation of evidence-based recommendations in clinical practice. Members' fields of practice include behavioral health, family medicine, geriatrics, internal medicine, pediatrics, obstetrics and gynecology, and nursing. Currently the Task Force is led by a Chair and two Vice-Chairs. Details on the roles and responsibilities of the Task Force members are provided in Appendix IV.
1.5.1 Selection of USPSTF Members
Each year, the Secretary of HHS selects new members to replace those members who are completing their appointments. Anyone can nominate a new Task Force member at any time on the Task Force Web site.
The nomination process and required qualifications are described on the Task Force Web site. As of December 2013, the required minimum qualifications are as follows.
Demonstrated knowledge, expertise, and national leadership in the following areas:
- The critical evaluation of research published in peer-reviewed literature and in the methods of evidence review.
- Clinical prevention, health promotion, and primary health care.
- Implementation of evidence-based recommendations in clinical practice, including at the clinician-patient level, practice level, and health system level.
Some USPSTF members without primary health care clinical experience may be selected based on their expertise in methodological issues, such as meta-analysis, analytic modeling, or clinical epidemiology. For individuals with clinical expertise in primary health care, additional qualifications in methodology would enhance their candidacy.
To obtain a diversity of perspectives, AHRQ particularly encourages nominations of women, members of minority populations, and persons with disabilities.
Applicants must have no substantial conflicts of interest, whether financial, professional, or intellectual, that would impair the scientific integrity of the work of the USPSTF and must be willing to complete regular conflict of interest disclosures.
Applicants must also have the ability to work collaboratively with a team of diverse professionals who support the mission of the USPSTF. Applicants must have adequate time to contribute substantively to the work products of the USPSTF.
1.5.2 Terms of Members
In 2001 the USPSTF transitioned to a standing Task Force. Currently, members are invited to serve for a 4-year term, with a possible 1-year extension. New members are selected each year to replace those who have completed their appointments.
1.6 USPSTF Meetings
The Task Force meets three times a year, in March, July, and November. Meetings are by invitation only. Representatives from USPSTF partner agencies and organizations have standing invitations. Special guests are invited to attend meetings for specific purposes.
Formal votes are taken for major procedural and methodological decisions, and for draft and final recommendations. Votes may be taken for other decisions at the discretion of the Chair. Detailed voting rules are provided in Section 7.4. Key provisions are as follows:
- All motions on recommendations (at any stage) requiring a vote are passed when two thirds of the current Task Force membership vote "yes."
- Motions on procedural, methodological, and other decisions which require a vote are passed when a majority of current Task Force membership votes "yes."
- Votes are submitted as "yes," "no," "abstain," or "absent." Votes are taken by voice, hand, or email, without secret ballots.
- Members recused for reason of potential conflict of interest are recorded as recused and do not vote.
- In votes that are less than unanimous, there are no minority reports.
A vote must be held to reconsider the grade of a previously voted draft or final recommendation statement. Two thirds of the current Task Force membership must approve the request to reconsider. If the request to reconsider is approved, the topic leads review and present the evidence supporting the motion. The Task Force then votes on the new recommendation either in person or by email.
1.7 Conflict of Interest
1.7.1 Introduction
The public must have confidence in the integrity of the process by which the Task Force makes its recommendations. The reputations of the Task Force members as highly regarded researchers, clinicians, and academicians contribute to this objective and must be protected if the Task Force recommendation statements are to be accepted and implemented. It is also essential that Task Force deliberations benefit from members' vigorous exchange of perspectives that are derived from and shaped by the member's research and/or practice experiences.
The intent of requesting disclosure of any potential conflict of interest is to ensure that the USPSTF provides a balanced, independent, objective, and scientifically rigorous product (including its recommendation statements) by understanding other interests that could potentially influence the work and decisionmaking of its members. The USPSTF requires each member to disclose all information regarding any possible financial and nonfinancial conflicts of interest prior to each meeting for all topics under development or that will be discussed at each meeting. Previous disclosures for continuing topics must also be updated to reflect changes in a member's situation since the form was last completed.
It is important to note that disclosures are not considered actual conflicts of interest until the value and nature of the disclosure is reviewed by the Task Force Chairs.
1.7.2 Process for Completing Disclosure Forms
The USPSTF Disclosure Form will be completed by Task Force members prior to each meeting to provide information on potential financial and nonfinancial conflicts of interest related to USPSTF topics under consideration. Task Force members are expected to provide full disclosure for new topics and topics in development, as well as an updated disclosure that reflects changes in their situation for continuing topics.
All members are expected to provide full disclosure of their own interests as well as the interests of immediate family members (which includes their spouse/partner, dependent children, and parents) and those of other close personal relationships.
The period of disclosure is 36 months prior to the date of form completion. The exception is publications related to the topic, for which there is no time limit, and research grants, for which the period of disclosure is 36 months from the end of the grant period. Completed Disclosure Forms will be kept on file. Further information on each type of disclosure required is provided below.
Disclosure of Significant Financial Interests
Financial disclosures refer to relationships with entities that could influence, or give the appearance of influencing, the outcome of a USPSTF decision. Entities could be individuals, organizations and corporations, or other groups with established or future business in the matter of a USPSTF decision. A relevant financial interest is a situation in which a Task Force member, immediate family member, or close personal relation has the potential for direct or indirect financial gain or loss related to a Task Force product. Task Force members should disclose financial relationships for themselves, their immediate family members, and close personal relationships. It is important to note that Task Force members report all relevant financial relationships regardless of the amount. Relevant financial interests include, but are not limited to:
- Ownership or owning individual stocks (stock shares, options, warrants), and bonds or other debt or other significant proprietary interests or investments in any third party that could be affected by a USPSTF decision on a specific topic. (Diversified nonsector mutual funds in which stocks are chosen by an independent fund manager may not need disclosure).
- Having an employment, independent contractor or consulting relationship or other contractual arrangements, whether written or unwritten, with an entity that could be financially or reputationally affected by a Task Force decision.
- Receiving a proprietary research grant or receiving patents, royalties, or licensing fees from such an entity.
- Participating on an entity's proprietary governing board or advisory council.
- Participating in an entity's speakers bureau.
- Receiving honoraria or travel from such an entity.
- Receiving payment as an expert witness for a plaintiff or a defendant associated with such an entity.
- Receiving remuneration for services with respect to transactions involving parties with a financial interest in the outcome of a USPSTF decision. This may include clinical specialty practice.
There is no set minimum dollar amount for financial disclosure because any relevant financial relationship could be considered significant.
Financial interests that do not need to be disclosed include:
- Income from seminars, lectures, teaching engagements, or service on advisory committees or review panels for public entities or nonprofit organizations that do not have a vested interest in the specified topics.
- Diversified mutual or retirement funds.
Disclosure of Significant Nonfinancial Conflicts of Interest
Nonfinancial conflicts of interest are other relationships, activities, or stated positions that could influence or give the appearance of influencing the work of a member of the USPSTF. In addition, nonfinancial conflicts of interest are considered to be any strongly held beliefs related to a topic area that would make it difficult for a Task Force member to work on any new or related topic. Task Force members should disclose these relationships, activities, or stated positions for themselves, their immediate family members, and close personal relationships. These disclosure requests are intended to identify strongly held opinions that may not be open to alternative conclusions even if provided with adequate evidence to the contrary. It also includes interests or institutional relationships that are not direct financial conflicts of interest but may influence or bias the individual.
The Task Force recognizes that potential nonfinancial interests are likely to be numerous because Task Force members are chosen for their national reputations on prevention issues; and their work may be very well known. As a result, users of Task Force products might doubt the objectivity of the process if such members are known to have taken leadership roles in discussion and vote on recommendations regarding that topic. Task Force members are required to disclose substantial nonfinancial interests including, but not limited to:
- Public comments and testimony.
- Leadership role on a panel.
- Substantial career efforts/interests in a single topic area.
- Previously published opinions.
- Advocacy or policy positions.
In addition, potential nonfinancial interests requiring disclosure include any relationships with or investments in governmental organizations, health care organizations, professional societies, or other organizations that you have reason to believe may benefit or be harmed by Task Force recommendations. This includes services that are provided on a part-time or seasonal basis, service that has occurred in the past or is anticipated in the future, and includes services for which compensation may have been provided as an:
- Officer
- Medical staff
- Board member
- Director
- Expert advisor
- Consultant
Nonfinancial Interests that do not need to be disclosed include:
- Employment from nonprofit organizations such as government agencies and nonprofit entities that do not have a vested interest in the specified topics.
- General membership in a professional society.
- Attendance at presentations or conferences related to the topic of interest.
Prospective Task Force Members
Prospective Task Force members will be verbally informed of the USPSTF conflicts of interest policy by the Task Force Chair and/or Vice Chair during the review of their candidacy. Appointees will be required to submit a Disclosure Form prior to finalizing their appointment. The USPSTF Disclosure Form will also be completed by new Task Force members prior to participation in their first in-person meeting.
1.7.3 Process for Determining Appropriate Actions
After disclosures are submitted and prior to each meeting or to new member appointment, all disclosures will be aggregated and reviewed by the Task Force Chairs. The Task Force Chairs will determine the final action on the member's eligibility to participate on a specific topic(s), which also is kept on file.
Each member is notified of the final action. If a Task Force member feels that a more conservative action is appropriate than that recommended, he or she can withdraw from any part of the process for that topic. For example, members are free to recuse themselves voluntarily from participation in the processes for specific topics. However, a voluntary recusal does not free a member from the obligation to disclose a conflict.
For disclosures and assessment of potential conflicts of interest of Task Force Chairs, the two Vice Chairs not under review determine a final action. This process is followed for each of the three Task Force Chairs.
Prior to each meeting, Task Force members will receive a summary of all disclosures that will be publicly announced during the meeting. At the start of each meeting, the Task Force Chairs will announce these disclosures and provide an opportunity for members to ask questions and engage in discussion.
Below is a list of disclosures representing potential conflicts and the possible actions that can be recommended by the Task Force Chairs for each disclosure.
Table 1. Description of Disclosures and Recommended Actions
| Level | Type of Disclosure | Range of Possible Recommended Actions | Description |
|---|---|---|---|
| 1 |
Financial Interests that do not need to be disclosed:
Nonfinancial Interests that do not need to be disclosed:
| No Action | No disclosure or recusal necessary |
| 2 |
| Information disclosure to Task Force only. | Member may participate as primary lead, and may discuss and vote on the topic |
| 3 |
| Possible exclusions from Task Force roles as a result of Level 3 disclosures Include: Member may not serve as primary lead for topic workgroup | Member may not participate as the primary lead of the topic workgroup specific to the conflict, but may serve as a lead on the topic workgroup and discuss and vote on the topic. |
| Member may not serve as the primary spokesperson for the topic | Member may not participate as the primary spokesperson for the topic specific to the conflict, but may serve as a lead on the topic workgroup and discuss and vote on the topic. | ||
| Member may not serve as a lead on the topic workgroup | Member may not participate as a lead in the topic workgroup specific to conflict, but may discuss and vote on the topic. | ||
| Recusal from all participation in topic activities | Member may not participate as a lead on the topic workgroup specific to conflict and may not discuss or vote on the topic. Member will leave the meeting room for all discussion and voting. Publicly released recommendations will denote the member's recusal from participation and voting on this topic. |
The member may choose to disclose to the Task Force chairs either a strongly held opinion that results in the potential for bias, or a personal or family illness that may lead to bias but which should be held confidential. This may result in recusal from a particular topic, at the discretion of the Task Force chairs.
If a relationship could be classified in more than one level (for example, service as a medical editor [Level 2] that is compensated at more than $1,000/year [Level 3]), it would be classified at the higher level (Level 3, in this case).
1.7.4 Process for Sharing USPSTF Disclosures and Actions With the Public
The USPSTF posts a summary of Level 3 disclosures for any topic on the conflicts of interest page of the USPSTF Web site. Additionally, International Committee of Medical Journal Editors (ICMJE) disclosure forms from USPSTF authors are available for each Recommendation Statement from the journal in which the Recommendation Statement is published.
Policy for Other Affiliated Groups
EPC members file separate disclosure forms consistent with EPC procedures and are kept on file.
1.9 Overview of the Process
As illustrated in Figure 1, four groups are involved in the process that results in formulating Task Force recommendations: the Task Force, AHRQ, the EPC, and Task Force partners. Each plays a unique role in the process.
The Task Force selects and prioritizes topics for review, approves the analytic framework, determines the questions and outcomes of interest, interacts with the EPC about evidence issues, judges and grades the level of the available evidence, determines the balance of benefits and harms, and makes the recommendation.
AHRQ convenes the Task Force and provides ongoing administrative, research, and technical support for its operations, including coordination of and support for the dissemination of recommendations. An AHRQ Medical Officer joins the topic team to provide technical input and assist with coordination. In addition, AHRQ staff occasionally prepares in-house evidence reviews for some update and reaffirmation topics (see Sections 2 and 4 for more information on reaffirmations).
Under contract to AHRQ, EPCs conduct systematic reviews of specified questions concerning the evidence on prioritized topics in clinical prevention. EPC evidence reviews serve as the scientific basis for USPSTF recommendations. The EPC's review process includes operationalizing the questions and outcomes of interest specified by the USPSTF for systematic review; drafting an analytic framework that illustrates the questions, populations, interventions, and outcomes of interest; locating and retrieving the relevant evidence; evaluating the quality of individual studies; qualitatively and/or quantitatively summarizing review findings for each question for use by the USPSTF in its evaluation of the evidence; and producing the reports. Further details about EPCs are available at www.ahrq.gov/research/findings/evidence-based-reports/overview/index.html
USPSTF partners are invited to review and comment on draft research plans, evidence reviews, and recommendation statements. Partners are encouraged to disseminate Task Force recommendations to their members. Further details about the role of partner organizations are provided in Section 1.8.
Lastly, anyone can nominate new Task Force members and new topics for the Task Force to consider. In addition, the USPSTF seeks feedback from the public on its draft research plans, evidence reviews, and recommendation statements.
The procedures for developing a recommendation statement are presented in Figure 2. A brief summary follows. Each step is also described in more detail in subsequent sections of the Procedure Manual.
1.9.1 Topic Selection
Topic selection begins with the identification of topics to be considered. Anyone—including individuals, organizations, EPCs, and Task Force members—can nominate a new topic for Task Force consideration or request an update of an existing topic through an online nomination form on the USPSTF Web site. Once a year, the Task Force Topic Prioritization Workgroup drafts a prioritized list of topics, including new topics and updates, to be started during that year. This list is made according to the following criteria for prioritization: public health importance (burden of suffering and potential of preventive service to reduce the burden); potential change to a prior recommendation (e.g., because new evidence has become available); and potential for a Task Force recommendation to affect clinical practice (based on existing controversy or the belief that a gap exists between evidence and practice). The ultimate goal is to balance the annual portfolio of topics by population, type of service (screening, counseling, preventive medication), type of disease (e.g., cancer, endocrine disease), and size of project (e.g., update vs. new topic). The Task Force also aims to update topics every 5 years in order to keep its library of recommendations current.
1.9.2 Work Plan Development
For every prioritized topic, a "topic team" is appointed. This team consists of three to four topic "leads" from the USPSTF membership, members of AHRQ staff (including a Medical Officer), and evidence review team members, including a lead investigator from the EPC. The responsibilities of the USPSTF leads are described in Appendix IV. Task Force members volunteer to be leads and are appointed by the USPSTF Chair. The EPC, working with AHRQ staff and Task Force leads (or in the case of an in-house evidence review, the AHRQ Medical Officer working with the Task Force leads) develops a preliminary work plan as described in Section 3. AHRQ organizes a conference call of the entire topic team to discuss and refine the project scope and finalize the work plan.
1.9.3 Work Plan External Review
Work plans for new topics are sent to a limited number of outside experts in appropriate areas for their review and comments. Work plans for topic updates are not routinely sent to experts for review.
1.9.4 Research Plan Development
Based on the full final work plan, a "research plan" that contains only the analytic framework, key questions, and inclusion/exclusion criteria is created for public comment.
1.9.5 Draft Research Plan Public Comment
All draft research plans are posted on the USPSTF Web site for public comment for a period of 4 weeks. USPSTF partners are encouraged to submit comments via the Web site.
1.9.6 Finalization and Approval of Work Plan
The work plan is revised based on public and partner comments and expert review. Work plans for new topics are usually presented by the EPC to the entire Task Force. The EPC's presentation is followed by comments from Task Force topic leads. The Task Force then discusses the plan, focusing on any issue of importance, but especially the key questions. The work plan is revised by the EPC as requested by the Task Force and finalized. Work plans for topic updates are approved by the Task Force topic leads, but are not routinely presented to the entire Task Force for discussion.
1.9.7 Draft Evidence Review
Based on the final work plan, the EPC conducts a systematic evidence review to address the questions posed by the Task Force and presents the resulting information in a draft evidence review, with evidence tables. The EPC presents a summary of the draft evidence review to the leads by teleconference before discussion and deliberation by the entire Task Force.
1.9.8 Review of Draft Evidence Review by Task Force Leads and External Experts
All draft evidence reviews are sent to a limited number of experts in the field for review (Appendix XV). In addition, Task Force topic leads and AHRQ Medical Officers are asked to comment on the draft evidence review.
Figure 1. Group Roles in the Task Force's Recommendation Development and Dissemination Processes
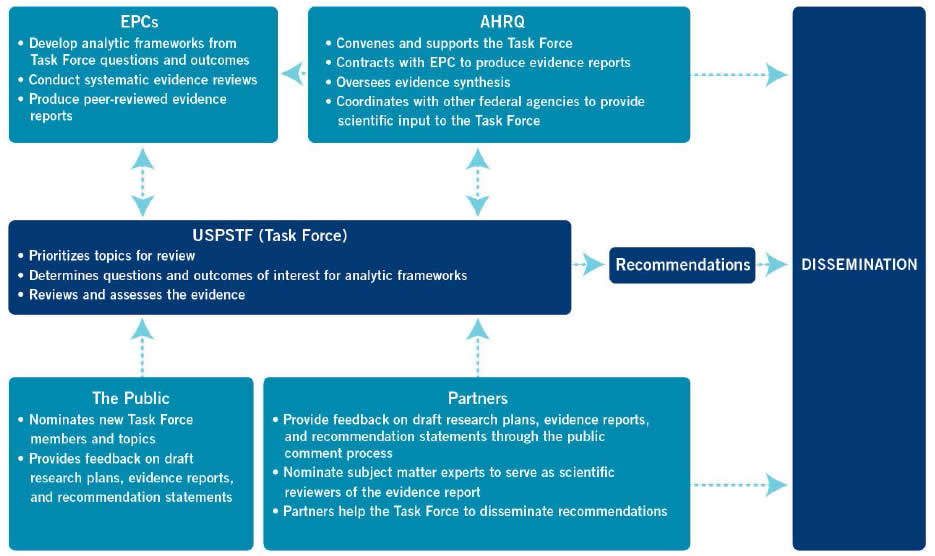
Figure 2. Steps the USPSTF Takes to Make a Recommendation

1.9.9 Development of Draft Recommendation Statement
While the draft evidence review is under review and revision, the Task Force topic leads discuss specific recommendations and the content of the Clinical Considerations section of the recommendation statement. The Task Force leads draft the recommendation statement with the AHRQ Medical Officer, which is presented to the entire Task Force at its next meeting.
1.9.10 USPSTF Vote on Draft Recommendation Statement
At the Task Force meeting, a representative from the EPC presents the expert-reviewed evidence review findings, and the Task Force topic leads discuss the evidence and present the draft recommendation statement. The entire Task Force discusses the evidence and recommendation statement. Any proposed changes to the specific language of the recommendation are discussed. The Task Force votes on various formulations of the recommendation statement until one version gains the support needed. It usually takes from 9 to 15 months from when the work plan is approved to when the peer-reviewed evidence review and draft recommendation statement are presented to the Task Force for a vote.
1.9.11 Public Comment on Draft Evidence Review and Draft Recommendation Statement
The draft evidence review and draft recommendation statement are typically posted together on the USPSTF Web site for public comment for a period of 4 weeks. During the comment period, any member of the public may submit comments on either or both of the documents. USPSTF partners are encouraged to submit comments.
1.9.12 Final Evidence Review
After receiving and reviewing all comments in the Draft Evidence Review from experts, partners, the public, the USPSTF (in particular, the topic leads), and the AHRQ Medical Officer, the EPC revises the evidence review. The EPC sends a summary of all comments received and the revised evidence review, indicating how the comments were addressed, to the AHRQ Medical Officer and made available to the Task Force. After the AHRQ Medical Officer has reviewed and approved the revised document, the review is considered final. At this point, the EPC may undertake preparation of a manuscript to be submitted for publication in a peer-reviewed journal. An effort is made to synchronize publication in the journal with the publication and/or release of the USPSTF final recommendation statement.
1.9.13 Development of Final Recommendation Statement
The Task Force leads working with the AHRQ Medical Officer propose revisions to the recommendation statement based on discussion at the meeting and all comments received from the public, experts, and partners. This revised recommendation statement is sent to all Task Force topic leads for approval.
1.9.14 Approval of Final Recommendation Statement
The final recommendation statement is then sent to all Task Force members for ratification, usually via email.
1.9.15 Release of Final Recommendation Statement and Final Evidence Review
An arrangement is made with appropriate journals to publish the final recommendation statement (which is published by the journal without substantive editing) and the manuscript derived from the EPC review. The desired timeline from USPSTF vote to recommendation release is 9 months. All final recommendation statements and supporting evidence are made available on the USPSTF Web site (www.uspreventiveservicestaskforce.org).
1.10 Procedures for Writing Papers and Documents
Task Force recommendations are usually published in a peer-reviewed journal. The Chair is listed as the author on behalf of the Task Force. Previous Task Force members who made significant contributions to the recommendation, such as leads on the topic workgroup who have since rotated off the Task Force, are also acknowledged. Members serving at the time of the recommendation's finalization are listed in an appendix to the publication.
Evidence summaries (articles summarizing evidence reviews produced by EPCs for each topic) are usually published in the same peer-reviewed journals as the corresponding recommendations. Authors include EPC staff contributors.
Additionally, the Task Force often disseminates its methods and processes through publication in peer-reviewed journals. When Task Force methods are shared through publication, clinicians and the general public can better understand the work of the Task Force and consider Task Force recommendations when making health care decisions.
Each individual designated as an author of a paper to be submitted to a journal should have participated sufficiently in the work to take public responsibility for the content. Authorship credit should be based on: 1) substantial contributions to the conception, design, analysis, or interpretation of data or literature; 2) participation in the drafting of the document or its revision for important intellectual content; and 3) giving final approval of the version to be published. All three conditions must be met, and all who qualify for authorship should be listed. USPSTF members who participate in the work but do not meet these criteria should be listed, with their permission, in the acknowledgments.
The Task Force does not recognize "courtesy" authorship given to Task Force members or EPC staff based on nominal role or position within a working group. General supervision of the working group, and participation in conference calls or group discussions, are not sufficient for authorship.
The Task Force works under severe time constraints in producing its products. Accordingly, Task Force members and EPC staff who wish to be authors should expect to provide component drafts, supporting materials, comment, and feedback on a timely basis to the lead author (a 1-week turnaround is a typical benchmark).
The order of authorship should be a joint decision of the coauthors. Because the order is assigned in different ways, its meaning cannot be inferred accurately unless it is stated by the authors. Authors may wish to explain the order of authorship in a footnote.
This policy is derived from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals, from the International Committee of Medical Journal Editors. This document is available at www.icmje.org
