The Task Force has a large library of current topics and frequently receives nominations for new topics. The overall goal for topic selection and prioritization is to provide accurate and relevant recommendations that are as up to date as possible and to balance the overall portfolio of recommendations by population, type of service (screening, counseling, preventive medication), type of disease (e.g., cancer, endocrine disease), and size of project (e.g., update vs. new topic). The Task Force also prioritizes topics with the aim of updating topics every 5 years, in accordance with currency criteria established by the National Guideline Clearinghouse, an AHRQ-initiated public resource for evidence-based guidelines. The criteria for new topic selection and for prioritization of active topics (discussed in detail below) are combined in an assessment of the topic as a whole, rather than used as part of a scoring system.
Table of Contents
- 2.1 Topic Types and Definitions
- 2.2 Determination of Scope and Relevance of New Topic Nominations and Topic Selection
- 2.3 Prioritization and Selection of Active Topics
- 2.4 Inactivating a Topic
- 2.5 Referring a Topic to Other Organizations
- 2.6 Consideration of an Early Topic Update
2.1 Topic Types and Definitions
There are two types of topics in the Task Force library: active and inactive. Among the active topics, there are four categories for consideration: new, updated, reaffirmed, and referred. The processes for developing work plans, assessing evidence, and making recommendations for active topics are discussed in Sections 3−7.
2.1.1 Active Topic Types
New topics are topics chosen by the Task Force for review and recommendation that have not been previously reviewed.
Updated topics are topics reviewed in the past by the Task Force that have since undergone an update of the evidence and recommendation. The update may encompass all key questions on a topic (full update) or only a limited set of the key questions in the analytic framework (targeted update).
Reaffirmed topics are topics kept current by the Task Force because the topic is within the Task Force's scope and a Task Force priority, and because there is a compelling reason for the Task Force to make a recommendation. Topics that belong in this category are well-established, evidence-based standards of practice in current primary care medical practice (e.g., screening for hypertension). While the Task Force would like these recommendations to remain active and current in its library of preventive services, it has determined that only a very high level of evidence would justify a change in the grade of the recommendation. Only recommendations with a current grade of A or D are considered for a reaffirmation evidence update. The procedure for a reaffirmation evidence update is discussed in Section 4.7.
Referred topics are topics in which the Task Force refers providers to another organization's recommendation. The Task Force originally made a recommendation on these topics and are retained as active in the Task Force library; however, the Task Force has determined that there is another organization (e.g., the CDC's Community Preventive Services Task Force, ACIP) with evidence-based methods that is better positioned to make accurate and timely recommendations for the topic. The procedure for referring to other organizations is discussed in Section 2.5.
2.1.2 Inactive Topics
Inactive topics are topics the Task Force has decided to not update or keep active for one or more reasons (go to Section 2.4 for more details).
2.2 Determination of Scope and Relevance of New Topic Nominations and Topic Selection
Anyone can nominate a new topic for Task Force consideration or request an update of an existing topic at any time online at https://www.uspreventiveservicestaskforce.org/uspstf/public-comments-and-nominations/nominate-recommendation-statement-topic.
Topic nominations are first considered by the Task Force's Topic Prioritization Workgroup, which then recommends selection and prioritization of new topics to the entire Task Force.
The Topic Prioritization Workgroup first considers whether newly nominated topics are within the scope of the Task Force, using the following criteria:
- The focus population should be asymptomatic for the condition of interest
- The nominated topic should represent a clinical preventive service (e.g., screening test, preventive medication, counseling about healthful behaviors)
- The preventive service should meet the definition of primary prevention (i.e., avoid the development of disease) or secondary prevention (i.e., identify and treat an existing disease before it results in significant symptoms)
- The preventive service should be provided in or referable from primary care
To further specify the situation that is the object of its concern, the Task Force has adopted the Institute of Medicine's definition of primary care:
Primary care is the provision of integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community. This definition acknowledges the importance of the patient-clinician relationship as facilitated and augmented by teams and integrated delivery systems.
The Task Force considers interventions that are delivered in primary care settings or are judged to be feasible for delivery in or referable from primary care. To be feasible in primary care, the intervention could target patients seeking care in primary care settings, and the skills to deliver the intervention are or could be present in clinicians and/or related staff in the primary care setting, or the intervention could generally be ordered/initiated by a primary care clinician.
Topics that are within the scope of the Task Force are then assessed for relevance using the following criteria:
- Public health importance (i.e., burden of suffering and expected effectiveness of the preventive service to reduce that burden)
- Potential for a Task Force recommendation to affect clinical practice (based on existing controversy or the belief that a gap exists between evidence and practice)
- Balance of Task Force portfolio (i.e., does the nomination overlap with current or in-process Task Force recommendations; does the nomination balance the overall Task Force portfolio of recommendations by population, type of service, type of disease, and/or size of project)
Based on the above criteria, the Topic Prioritization Workgroup assigns each nomination to one of the following categories for consideration by the entire Task Force:
- Not a potential new topic:
- Out of scope
- In scope, of less relevance
- In scope, already addressed
- In scope, potential new topic
The entire Task Force ultimately votes on the selection of potential new topic nominations for inclusion in the Task Force portfolio. As new topics are selected, the Topic Prioritization Workgroup and full Task Force prioritize the potential new topics in comparison with existing new topic nominations. The Task Force maintains a list of one to three new topic nominations for possible review over the next 2 years. All potential new topics enter the yearly prioritization process (described in Section 2.3).
2.3 Prioritization and Selection of Active Topics
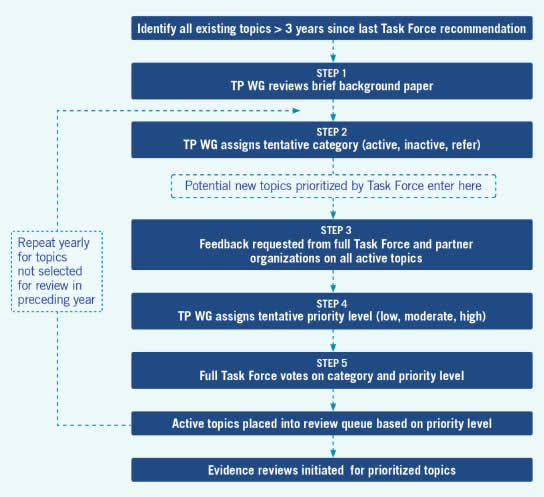
The Topic Prioritization Workgroup begins prioritization of an active group of topics approximately 3 years after their previous publication.
Step 1. A brief background paper on the topic is produced that includes the following information: previous recommendation statement, estimate of disease burden, relevance to prevention and primary care, recommendations of other guideline developers, existing controversy or gap between evidence and practice, and summary of a brief literature search for new evidence.
Step 2. The Topic Prioritization Workgroup reviews and discusses the background paper and places each topic into either the active or inactive category. Topics that are retained as active are considered for referral to other organizations (go to Section 2.5 for the process of referring a topic and Section 2.4 for the process of inactivating a topic).
Step 3. A request for feedback on all active topics and potential new topics is sent to Task Force members and partner organizations. Respondents are asked to categorize each proposed topic as high-, moderate-, or low-priority for review in the next 12 to 18 months, based on the following criteria:
- Public health importance (i.e., burden of suffering and expected effectiveness of the preventive service to reduce that burden)
- Potential for a Task Force recommendation to affect clinical practice (based on existing controversy or the belief that a gap exists between evidence and practice)
- New evidence (e.g., new studies or new analyses of previous data) that has the potential to change the prior recommendation
- Need for a balanced portfolio of topics
Step 4. The feedback from Task Force members and partner organizations is considered by the Topic Prioritization Workgroup, along with the background paper, in assigning a tentative priority category for active topics. The four criteria listed in Step 3, along with resource requirements for the review, are used to recommend priority (low, moderate, or high).
Step 5. The topic categorization (active, inactive, refer) and prioritization (high, moderate, low) becomes final after a vote of the full Task Force membership.
Steps 2 to 5 are repeated yearly for topics not selected for review in the preceding year (Figure 3).
AHRQ staff develops the work queue for the next 12- to 18-month cycle using the priority level determined by the Task Force. Other factors that may be used by AHRQ staff in determining the work queue include: availability of research team, availability of review or funds from a non-USPSTF source, efficiency of combining reviews or research teams on related topics, impending release of relevant study, and age of relevant non-USPSTF review.
2.4 Inactivating a Topic
Inactive topics are topics the Task Force has decided to inactivate for one or more of the following reasons:
- Topic is no longer relevant to clinical practice because of changes in technology, new understanding of disease etiology/natural history, or evolving natural history of the disease
- Topic is not relevant to primary care because the service is not implemented in a primary care setting or not referable by a primary care provider
- Topic has a low public health burden
- Topic is otherwise outside of the Task Force's scope
Previously inactivated or referred topics are also eligible as new topic nominations, if appropriate, along with other new topic suggestions.
If a topic is inactivated or referred to another organization, the status on the Task Force Web site continues to be listed as "active" for a minimum of 5 years from the date of the original recommendation, unless considerations arise beforehand to change the status. After this period, the status changes to "inactive" or "referred."
2.5 Referring a Topic to Other Organizations
Recommendations for some topics in the Task Force library may be referred to another organization that the Task Force believes is in a better position to make an accurate and timely evidence-based recommendation. This practice avoids redundancy of resource use by the Task Force. An example is ACIP, a non-Federal panel of immunization experts convened by the CDC. In the past, the Task Force has referred recommendations on immunizations to ACIP. Another example is the CDC-supported Community Preventive Services Task Force, which makes evidence-based recommendations on many health promotion topics.
The organization identified for referral should have the resources for timely updates of the evidence and a scientifically acceptable methodology for its evidence reviews (see the list of criteria below). The process for designating a topic for referral is as follows:
- The Topic Prioritization Workgroup identifies a potential outside organization that makes evidence-based recommendations and decides to consider the topic for referral.
- The Topic Prioritization Workgroup reviews the previous Task Force recommendation statement and evidence review.
- The Topic Prioritization Workgroup reviews the recommendations and review methods of the chosen organization.
- A brief summary is prepared that includes why the topic has been chosen for referral, a reference to the chosen organization's recommendations on the topic, a statement that the organization's methodology may be different from the USPSTF's, a new recommendation date, and a statement that the previous evidence review will not be updated.
- The Topic Prioritization Workgroup decides whether to proceed with a full Task Force discussion.
- If the Topic Prioritization Workgroup decides to proceed, the summary is presented at a Task Force meeting for general discussion. The Task Force then votes on the decision to refer the topic to the specific organization.
- A single summary paragraph is added to the USPSTF Web site that includes a link to the organization's recommendation.
The criteria for referring to another organization's recommendation are:
- The organization has been identified by the Task Force as an appropriate source
- The organization has a process for updating recommendations in a timely manner
- The organization has a written and available evidence-based methodology, including the use of systematic reviews that assess benefits and harms, that the Task Force judges to be adequate for the topic
Referred topics may be reactivated through the usual new topic nomination process (described in Section 2.2).
Figure 3. Steps in Topic Prioritization
2.6 Consideration of an Early Topic Update
Occasionally a study will be published after a recommendation's release that may potentially affect the Task Force's consideration of the evidence and its conclusions about the certainty and/or magnitude of the net benefit (and the recommendation itself). These studies are brought to the attention of the Task Force by a number of sources, including the public, Task Force members, EPCs, professional organizations (including Task Force partners), and advocacy groups.
A regular audit of information sources is conducted to locate newly published research and/or guidelines that are relevant to topics in the Task Force portfolio. This LitWatch process is described in Appendix III. The Task Force uses the following process to consider new evidence and decide whether a recommendation needs to be updated earlier than the usual 5-year timeframe:
- The AHRQ staff member or another assigned Medical Officer completes a form with the following items:
- Citation
- Nominator and affiliation
- Assigned Medical Officer
- Brief summary/abstract of study
- Number of criteria met (see below)
- Recommendation of Medical Officer/Scientific Director
- Summary of Topic Prioritization Workgroup and Task Force discussion (to be completed later in the process)
- Action/disposition (to be completed later in the process)
- The Medical Officer proposes a disposition as to whether the new evidence should trigger an early review, based on the following criteria (order is not necessarily based on criteria weighting):
- New evidence conflicts with current recommendation
- Large-scale study may improve certainty of net benefit
- New evidence has potential to change recommendation grade
- Evidence focuses on a new intervention/strategy not previously considered
- Study shows a change in magnitude of benefit or harm that might alter the Task Force's assessment of magnitude of net benefit
- Evidence has the potential to fill a gap in the chain of indirect evidence
- High level of existing controversy about the topic
- High public health burden of the condition
- High quality or relevance (e.g., a randomized, controlled trial [RCT] is published on a topic for which the current recommendation is based on observational evidence)
- Published in a peer-reviewed journal
- Study directly links the prevention strategy to the primary outcome of interest (i.e., direct evidence of health effect)
- Study was identified by a reliable source (e.g., professional organization, Task Force member, advocacy group)
- The form and the Medical Officer's recommendation are sent to the Scientific Director and the AHRQ lead in the Topic Prioritization Workgroup.
- If appropriate, a discussion of the evidence is placed on the agenda for the Topic Prioritization Workgroup's monthly conference call. If there is an identified current Task Force member who is a topic lead or expert in the subject area and who is not a member of the Workgroup, then that Task Force member is invited to participate in the conference call. The evidence and the review form are sent to the call attendees with an agenda.
- The Topic Prioritization Workgroup discusses the evidence and, using the criteria defined above, makes a recommendation to the entire Task Force about whether the evidence should trigger an early update of the review.
- The Task Force votes at its next meeting on whether the evidence update should be accelerated because of the new evidence. If the Task Force votes for an early topic update, the Task Force also assigns a priority level (high, moderate, low) based on the usual topic prioritization criteria.
- If the Task Force decides to accelerate the update, the USPSTF Scientific Director at AHRQ places the topic in the review queue.
A brief notice from the Task Force Chair is sent to the nominator about the disposition.
